Case guesses:
Wink Weinberg writes:
Dear TWIP Professors,
I really do not know what caused the cardiac birth defects in the mother who returned to Brazil in her third trimester. However, according to the following article, congenital infection with Trypanosoma cruzi is a real hazard in Bolivia. Furthermore, congenital transmission occurred predominantly in newborns with a gestational age of 26 to 37 weeks.
TWIP is my favorite of all your podcasts and of all of the medical podcasts in my queue. (One birding show and one financial show come sort of close.) Thanks for all the work you put into TWIP.
Wink Weinberg
(By the way, those other podcasts are Ray Browns Talkin’ Birds and Motley Fool Money.)
Trans R Soc Trop Med Hyg. 1985;79(2):176-80. Congenital Chagas’ disease in Bolivia: epidemiological aspects and pathological findings.
Alan writes:
Estimados Profesores TWIP,
We are done with this year’s hurricanes and volcanoes and are enjoying nearly forgotten fall Kona weather.
For the Case Study for TWiP 161, a 30 yo female from Bolivia, who lived in Bolivia during her 3rd trimester and subsequently delivered a child with pericardial effusion, ascites, and moderate PDA:
I’ve had the privilege to work with the wonderful people of the Bolivian altiplano from 1992-1993, and anytime you have cardiac symptoms from this region, you have to be suspicious of Chaga’s disease (American Trypanosomiasis); in this case congenital chagas, which while rare in the US, is not un-common in Bolivia.
Confirmation:
The most sensitive test for to confirm congenital and acute Chagas disease is the T cruzi PCR assay. Possible false positives from the mother’s antibodies can be reduced by repeating an early positive PCR test when the infant is over 9 months. Of course spotting T cruzi trypomastigotes in cord blood or in the infant’s blood would confirm the diagnosis but that’s often much less sensitive than molecular detection.
Early treatment of acute congenital infection can have a cure rate of greater than 90% and can prevent severe complications of chronic Chagas disease.
Either benznidazole or nifurtimox can be used for treatment, and both of them are generally well tolerated.
However, a good differential would have to include Trichinella spiralis, Toxoplasmosis, filariasis, amebic pericarditis – perhaps as a complication an amebic liver abscess, Hydatid disease, and beyond parasites, perhaps cytomegalovirus, herpes simplex virus and rubella.
Second guessing myself:
Ascites often means cirrhosis. Have seen a lot of ascites in the southern Philippines from Schistosoma japonicum, and from Strongyloidiasis from S. fuelleborni in Sub Saharan Africa, but neither live in Latin America. Amebic liver abscess and amebic pericarditis would possibly have leukocytosis, but probably not eosinophilia.
Patent ductus arteriosus (PDA) could develop simply from living at high altitude (above 3,000 meters or 10,000 feet) during the third trimester, as much of the Bolivian altiplano is; or from the mother having rubella during pregnancy, but rubella is not a TWIP parasite. Soooooo,
I’m sticking with Chagas Disease.
Again, thank your for your continued investment of your time and passion in producing these wonderful podcasts.
Saludos,
Allan
Kona, Hawaii
Allan Robbins, DIH, MPH
University of the Nations
Global Health Training
Kailua-Kona, HI
Kevin writes:
Hydrops keep fallin’ on my head
The Bolivian neonate with a pericardial effusion, ascites, and a patent ductus arteriosus. Elevated WBC, slight lymphocytosis, normal eosinophils. Mother has been traveling back and forth from USA and Bolivia, most recently during the third trimester.
A case redolent of congenital parasitosis.
The condition of fluid accumulation in at least two fetal body cavities is defined as hydrops fetalis. The word hydrops comes from the Greek word for water, and is also synonymous with the old fashioned term ‘dropsy’. Shakespeare drops the word dropsy in a wonderful catalog of insults directed by Prince Hal at Falstaff: “Why dost thou converse with that trunk of humors, that bolting-hutch of beastliness, that swollen parcel of dropsies, that huge bombard of sack, that stuffed cloakbag of guts…” Shakespeare, Henry VI-1, 2:4
Consult my notes and references for a further fatiguing discourse about the terms hydrops and dropsy.
Hydrops fetalis is a syndrome, an aggregation of clinical signs that are the end result of multiple specific diseases. Historically the most common cause of hydrops was Rh disease, which was greatly reduced by the introduction of Rh immunization (RhoGam) beginning in 1968. Immune related hydrops still occurs, most frequently in alpha thalassemia major with hemoglobin Barts. Non-immune hydrops fetalis (NIHF) is now the most common category of this condition. The mechanism of fluid accumulation is complex; consult the below references if you want to dig deeper into this watery topic. There are multiple causes of NIHF, but the one that concerns us here is infection. A variety of congenital viral and bacterial infections can cause NIHF, most commonly parvovirus B17 and CMV. Less frequently, fetal infection with syphilis, tuberculosis and listeria can result in hydrops. Now that the preliminaries are disposed of, I will move into parasitic infections that can potentially result in a clinical scenario similar to our Bolivian baby.
Possible causes of congenital parasitosis
Entamoeba histolytica: congenital infections have been described. I can find no evidence that this infection causes hydrops.
Malaria: According to the CDC Yellow Book there is malaria transmission in Bolivia (culprits: P. vivax 93%, P. falciparum 7%). Hydrops due to congenital malaria has been described (see REF) but is extremely uncommon. Neonates with congenital malaria are usually healthy at birth (REF). The mother in our case is reported to be well, so we can reasonably exclude malaria as a consideration.
Leishmania (visceral)- Though Bolivia is reported to have the highest incidence of cutaneous leishmaniasis in the world, visceral leishmaniasis (Leishmania chagasi) is rare in Bolivia. Autochthonous cases have been described but are more common in Brazil (see Desjeux ref). Congenital visceral leishmaniasis has been reported from Brazil but is uncommon and I could no literature on associated hydrops. I think that this diagnosis is unlikely for our case.
African trypanosomiasis- Note that this can cause congenital infection. Not relevant here.
Trichinella: rare cases of prenatal transmission have been reported. Larva are transmissible via breast milk.(Moldanado). Mentioned here as a nod to Dr Despommier; otherwise of no relevance to our case. Also, there is no eosinophilia
Schistosoma: Placental infection has been described in up to 25% of pregnancies in endemic areas. Fetus is generally unaffected. (Moldanado ref). Besides, there are no schistosomes in Bolivia. (Though an indigenous potentially competent snail vector has been found there.)
Other: a host of helminths have been described as being transmitted from mother to fetus, but are not relevant to our case and I could find nothing describing an association with hydrops. (See 2011 Dotters-Katz review and misc refs below)
Congenital infections most relevant for our case
Trypanosoma cruzi: Carlier’s 2002 review states that maternal-fetal transmission of T. cruzi ranges from 2-12% of pregnancies in Bolivia, Argentina, Chile and Paraguay. Hydrops has been described but seems to be quite uncommon. Though congenital Chagas disease is an important cause of neonatal morbidity, the clinical presentation usually described as subtle or asymptomatic, however, up to 10% of vertically transmitted infections can manifest symptoms in the neonate. Screening infants in endemic areas is importance since congenital infection has the same potential to cause chronic disease as vector acquired infection. Diagnosis: serology, Western blotting, PCR, direct parasite visualization in blood or tissue (which have poor sensitivity). Rapid diagnostic tests: an indirect hemagglutinin test and TrypanosomaDetect, immunochromatographic strip assay. Accurate and high sensitivity diagnosis however remains a problem in low resource countries. Treatment is benznidazole (though only FDA approved for ages 2 and over) and nifurtimox (available through the CDC). According to the CDC cure rates in infected infants exceed 90%. TWiP case 161 is similar to one reported in MMWR in 2012, where a 31 y/o Bolivian woman living in Virginia gave birth (Caesarian) to a child with ascites, pleural and pericardial effusions (described as fetal hydrops). Other relevant infectious diseases were ruled out. T. cruzi was seen in the neonate’s peripheral blood. This was the first reported case of congenital Chagas disease in the United States. Congenital Chagas disease is a strong contender for the cause of our patient’s clinical presentation.
Toxoplasmosis: I could not locate epidemiologic data regarding congenital toxo in Bolivia, but a review from 2000 estimated that prevalence in Brazil is 1/3000 live births. Fortunately, the majority of these laboratory detected infections were subclinical. (The relatively high Brazilian prevalence is compared to Massachusetts, where the estimate was 1/10,000 live births.) A large 10 year Brazilian survey states that the incidence of symptomatic congenital toxoplasmosis was 5/10,000 (REF). Most cases (over 75%) of fetal toxoplasmosis are asymptomatic /subclinical, but when affected, the fetus may show CNS disease, intracranial calcifications, chorioretinitis, and systemic disease with hepatosplenomegaly, lymphadenopathy, hyperbilirubinemia, seizures, rash, myocarditis, pneumonitis, an erythroblastosis picture (erythroblastosis is a severe neonatal hemolytic anemia-like Rh disease- that can result in hydrops fetalis). Lymphocytosis has been described. Diagnostic screening is by dot/blot IgM test; PCR is used in antenatal diagnosis. Treatment is given over 12 months and includes pyrimethamine, sulfadiazine, and folinic acid.
Congenital toxoplasmosis is another tempting choice as the culprit in our case.
Wrap-up: The paucity of clinical data given in this case makes choosing a diagnosis difficult. Nevertheless, due to the absence of widespread disease signs and symptoms- (so called TORCH syndrome =toxoplasmosis, other agents, rubella, cytomegalovirus, and herpes simplex) leads me to dismiss congenital toxo. Despite the infrequent occurrence of symptomatic congenital Chagas disease (as well the almost non-existence of case reports from the US), I will submit congenital Chagas as the cause of disease in baby TWiP 161.
A final word:
Patent ductus arteriosus: present in ~30% of pre-term infants. Besides prematurity, PDA is also associated with congenital heart malformations, genetic disorders, also associated with a variety of prenatal infections such as rubella, chorioamnionitis, tuberculosis. Untreated, PDA can result in pulmonary hypertension and heart damage. It is treated with indomethacin and other non-steroidal anti-inflammatory drugs (which here act via prostaglandin inhibition). Drug resistant PDA can be managed surgically. I suggest that PDA in our patient is a fellow traveller, an epiphenomenon of the overall infection. I could find no specific parasitic disease associations with PDA.
Forgive these lucubrations, and thanking TWiP professors for their patience.
REFERENCES:
including—> Vocabulary, Comments and a Terminal Curiosity from Olden Times
1 Some vocabulary and definitions:
**Hydrops fetalis is defined as the presence of excessive fluid accumulation in at least two fetal body cavities; including ascites, pleural effusion, pericardial effusion, and skin edema; often associated with polyhydramnios and placental edema. Shipra Nigam, International Journal of Reproduction, Contraception, Obstetrics and Gynecology Nigam S et al. Int J Reprod Contracept Obstet Gynecol. 2016 May;5(5):1640-1642
**Massive edema, pleural and pericardial effusions and peritoneal spaces characterize hydrops fetalis.
A review on TORCH: groups of congenital infection during pregnancy Rajnish Kumar Yadav, Journal of Scientific and Innovative Research 2014; 3 (2): 258-264 open access
**Fetal hydrops was defined as the presence of fluid collection in ≥2 body cavities: ascites, pleural effusion, pericardial effusion, and skin edema. Clinical characteristics and perinatal outcome of fetal hydrops, Wonkyung Yeom et al, Obstet Gynecol Sci. 2015 Mar; 58(2): 90–97. This is a comprehensive, open access review of the topic with an extensive reference list.
**The term hydrops fetalis describes the excessive fetal fluid accumulation in at least two serous cavities (abdomen; pleura; pericardium) or in body tissue (subcutaneous edema) Nonimmune hydrops fetalis: A short review of etiology and pathophysiology, Bellini C, et al Am J Med Genet 2012 Part A 158A:597– 605. -This review goes into insane detail in explaining the mechanism of these pathologic fluid accumulations.
Hydrops: “a preternatural collection of a serous fluid in any cavity of the body, or in the areolar texture. Dunglison’s Medical Dictionary, Robley Dunglison MD, LL.D, Blanchard and Lea: Philadelphia, 1860
John Whitridge Williams 1912 textbook “Obstetrics”, employs the term “general dropsy of the fetus” -also the title of Ballantyne’s famous 1892 monograph (open access).
I could find the word ‘hydrops’ used as early as 1805 in Jahresbericht Geburtshilfe und Gynaekologie.
Hydrops and dropsy are used interchangeably in Edgar’s 1912 The Practice of Obstetrics…
The term ‘dropsical effusions’ has been used in old medical works to describe effusions of the pericardial, pleural and peritoneal variety.
The Oxford English Dictionary defines hydrops as ‘a dropsy’ and the second usage is dated 1706: Hydrops: The Dropsie, from the Greek hydor, hyd- = water (think HYDration)
From Bartholome Parr, The London Medical Dictionary 1819, HYDROPS: A dropsy — All dropsies are chronical diseases from debilitated fibres; but this debility may be general or particular. It is, however, always attended with an accumulation of serosity, either in the whole of the cellular texture, or in particular cavities. In the anasarca the water is clear and limpid, but in the ascites often more thick and gelatinous, or sometimes mixed with hydatids or coagulated lymph. Hydrops pericardii Hydrops abdominis-ascites
“NIHF should be considered a non-specific, end-stage status of a wide variety of disorders [Bellini et al., 2009a]
2 Some miscellaneous facts about hydrops:
Laundry list of infectious causes of non-immune hydrops fetalis (source: Medscape.com):
- Parvovirus B19V
- CMV
- Syphilis
- Herpes simplex
- Toxoplasmosis
- Hepatitis B
- Adenovirus
- Ureaplasma urealyticum
- Coxsackievirus type B
- Listeria monocytogenes
- Enterovirus
- Lymphocytic choriomeningitis virus (LCMV)
15-20% of non-immune fetal hydrops=no etiology found (2013 SOGC guidelines)
A 2017 study of NIHF from southern China looked at 427 cases and only 7 of these were related to infection, primarily parvovirus B19 and CMV.
3 Medical Literature References (with relevant quotes and a few editorial comments):
Investigation and Management of Non-immune Fetal Hydrops Valérie Désilets, MD, J Obstet Gynaecol Can 2013;35(10):e1–e14
Etiology and Prognosis of Fetal Ascites,Annette Schmider et.al, Fetal Diagn Ther 2003;18:230–236
Vintage reference: NEJM review of hydrops from 1966- nice list of all the causes of hydrops—-
Hydrops Fetalis, Shirley G. Driscoll, M.D. December 22, 1966 N Engl J Med 1966; 275:1432-1434
A list of conditions that have been associated with fetal hydrops follows: hemolytic disease of the newborn; fetal maternal hemorrhage; alpha-thalassemia; twin transfusion syndrome (discordant hydrops, usually affecting the recipient twin); multiple gestation, with 1 “parasitic” fetus (hydrops affecting the parasitic twin, but perhaps also the “normal” twin); achondroplasia; cystic adenomatoid malformation of the lung; pulmonary lymphangiectasia, with or without other anomalies; cardiopulmonary hypoplasia, with bilateral hydrothorax; multiple congenital anomalies; maternal diabetes mellitus; dysmaturity; toxoplasmosis; cytomegalovirus disease; syphilis; leptospirosis; Chagas’s disease; congenital hepatitis; fetal neuroblastomatosis; congenital nephrosis; renal-vein thrombosis; chorionic-vein thrombosis; umbilical-vein thrombosis; and finally, no apparent cause (idiopathic).
Society for Maternal-Fetal Medicine(SMFM) Clinical Guideline #7:nonimmune hydrops fetalis, Mary E. Norton, MD et al, FEBRUARY 2015 American Journal of Obstetrics & Gynecology
A simple and easily digestible review article. Good table (Table 1) outlining the etiologies and mechanisms of nonimmune hydrops. Infection is listed as causing 5-7% of cases, with the following mechanisms of pathologic fluid accumulation: anemia, anoxia, endothelial cell damage, and increased capillary permeability. Their definition: hydrops fetalis is a Greek term that describes pathological fluid (“ὕdur,” Greek for water) accumulation in fetal soft tissues and serous cavities. The features are detected by ultrasound, and are defined as the presence of 2 abnormal fluid collections in the fetus. These include ascites, pleural effusions,pericardial effusion, and generalized skin edema (defined as skin thickness >5 mm).
Ultrasound of Congenital Fetal Anomalies: Differential Diagnosis and Prognostic Indicators, Second Edition Dario Paladini, Paolo Volpe, CRC Press, Mar 29, 2018
“NIHF is a nonspecific sign of various infections vertically transmitted from mother to fetus, including both viral and nonviral infections… mechanisms: inflammation, myocarditis with pump failure, hemolytic anemia and or hepatitis, and hepatitis induced hypoproteinemia. The final result of the various conditions mentioned above is a breakdown of equilibrium between intracapillary and extracapillary pressures, with consequent fluid ultrafiltration in the interstitial space. “
Hydrops Revisited: Literature Review of 1,414 Cases Published in the 1980s, Geoffrey A. Machin, American Journal of Medical Genetics 34366 -390 (1989)
“The diseases that cause fetal and neonatal ascites have also recently been summarized [Machin, 1981. In some reports, notably in the radiologic literature, hydrops and ascites are terms used almost indiscriminately; although ascites (together with pericardial effusion) is often an early manifestation of serous fluid accumulation in fetuses who later develop full-blown hydrops, there are also many causes of ascites that are not systemic in nature, but are caused by defects localized in the abdomen. It should also be recalled that ascites and pleural effusions may actually cause hydrops rather than vice versa.”
Etiology and Perinatal Outcome of Nonimmune Hydrops Fetalis in Southern China, Sheng He, PhD, AJP Rep. 2017 Apr; 7(2): e111–e115.
Paper is a review of 482 cases, only 7 were of infectious etiology (parvovirus B19 and CMV).
Non-Immunologic Hydrops Fetalis: Study of 86 Autopsies, Garcia, A. et al (1996).. Tropical Doctor, 26(2), 78–79. 1996.
Case series of 38 years. 31=syphilis, 30=”intrauterine hematogenic infection of unknown etiology”, rubella, parvovirus, toxoplasma, twinning, genetic
Investigation and Management of Non-immune Fetal Hydrops, SCOG GUIDELINES, Valérie Désilets, MD, et al, J Obstet Gynaecol Can 2013;35(10):e1–e14 open access
Of 5,437 cases of fetal hydrops reviewed, 6.7% were due to infections. Most infections due to parvovirus B19. Other infections: toxoplasmosis, syphilis, CMV, varicella
Infectious Causes of Hydrops Fetalis, Barron, Steven, et al, Seminars in Perinatology, 19:6 (Dec) 1995,493-501
Discussion restricted to Parvo B19, HSV, CMV, toxoplasma, Treponema pallidum (an accompanying table also mentions coxsackie, adenovirus, polio,rubella, flu B, RSV,Listeria, Leptospira,T. cruzi, Chlamydia, Ureaplasma. Mechanisms of hydrops generation: infection of erythroid precursors, direct hepatic damage, myocarditis.
Impact of Fetal and Neonatal Viral (and Parasitic) Infections on Later Development and Disease Outcome, Yvonne A. Maldonado, Barker DJP, Bergmann RL, Ogra PL (eds): The Window of Opportunity: Pre-Pregnancy to 24 Months of Age. Nestlé Nutr Workshop Ser Pediatr Program, vol 61, pp 225–242, 2008
The Association of Parasitic Infections in Pregnancy and Maternal and Fetal Anemia: A Cohort Study in Coastal Kenya, Elizabeth M. McClure, PLoS Negl Trop Dis. 2014 Feb; 8(2): e2724. [doi:10.1371/journal.pntd.0002724 Published online 2014 Feb 27.]–open access
no mention of hydrops, abnormal fluid collections.
Congenital parasitic infections: A review, Yves Carlier et al, Acta Tropica 121 (2012) 55– 70
Parasites known to be congenitally transmitted from infected pregnant women to their fetuses are mainly protozoa (Toxoplasma gondii, Trypanosoma cruzi and Plasmodium spp., and occasionally, Trichomonas vaginalis, African trypanosomes, and agents of visceral
leishmaniasis), whereas the transmission of helminths rarely occurs in humans. Signs and symptoms of parasitic congenital infections are generally similar to those of other common congenital infections, such as those due, e.g. to cytomegalovirus and Herpes simplex virus. T. gondii, T. cruzi or Plasmodium-infected newborns frequently exhibit fever, low birth weight (<2500 g), prematurity (gestational age < 37 weeks), hepatosplenomegaly, jaundice, and pneumonitis. The word ‘hydrops’ does not occur in this review
Parasitic Infections in Pregnancy, Sarah Dotters-Katz, MD,Volume 66, Number 8 OBSTETRICAL AND GYNECOLOGICAL SURVEY 2011
Review that includes everything but the proverbial kitchen sink. Sadly, the word ‘hydrops’ never makes an appearance.
Human visceral leishmaniasis in Bolivia: first proven autochthonous case from ‘Los Yungas’, P. Desjeux, Transactions of the Royal Society of Tropical Medicine and Hygiene 1983 , Volume 77 , Issue 6 , 851 – 852
Congenitally transmitted visceral leishmaniasis: report of two Brazilian human cases, Myrlena Regina Machado Mescouto-Borges et al, Braz J Infect Dis vol.17 no.2 Salvador Mar./Apr. 2013
Uncommon Association Between Chronic Malaria and Hydrops Fetalis, Saeed Mohamed Ahmed Thabet, MD, Annals of Saudi Medicine, Vol 4 No. 4; 1984
Review of 11 cases. The neonates did not have detectable plasmodium in their blood. Case series from southern Saudi Arabia and Cairo Egypt. All had ascites and enlarged placentas. The authors state that “malaria has not been previously investigated as a cause of hydrops fetalis.
Congenital malaria in a neonate: case report with a comprehensive review on differential diagnosis, treatment and prevention in Indian perspective, Preeti Rai, J Parasit Dis. 2015 Jun; 39(2): 345–348.
Neonatal ascariasis, Chu, W. et al (1972).. The Journal of Pediatrics, 81(4), 783–785. doi:10.1016/s0022-3476(72)80103-3
Interesting case report but nothing about hydrops
Diagnosis, Treatment, and Prevention of Congenital Toxoplasmosis in the United States Yvonne A. Maldonado, Pediatrics February 2017, VOLUME 139 / ISSUE 2
Toxoplasmosis, Ruth Lynfield, Nicholas G. Guerina, Pediatrics in Review March 1997, VOLUME 18 / ISSUE 3
Congenital Toxoplasmosis, James B. McAuley Journal of the Pediatric Infectious Diseases Society, Volume 3, Issue suppl_1, 1 September 2014 open access
An open access, readable, solid clinical review.
Congenital toxoplasmosis in South American children, Jorge E. Gómez-Marín, Scientia Medica (Porto Alegre) 2010; volume 20, número 1, p. 103-107
The frequency of congenital toxoplasmosis as determined by newborn screening programs in South America varies in rates of 0.6% reported in Colombia to 0.01% in some regions of Brazil. This frequency is at least 10 times higher in most of the studies performed in South America than in those reported in Europe and United States. The author also states that congenital toxo is more severe with more abnormalities (i.e. ocular and intracranial) reported in the South American cohort.
Incidence of Symptomatic Congenital Toxoplasmosis During Ten Years in a Brazilian Hospital, Bischoff, Adrianne Rahde MD, The Pediatric Infectious Disease Journal: December 2016 – Volume 35 – Issue 12 – p 1313–1316.
This large study from a pediatric university hospital looked at all synmptomatic congenital cases over a ten year period. The mean incidence was 5 symptomatic cases/10,000.
Prenatal diagnosis of congenital Chagas’ disease (American trypanosomiasis), Okumura M et al,Prenat Diagn. 2004 Mar;24(3):179-81.
Case report. Neonate had ascites, hydrocele. Mentions Bittencourt’s 1975 case series of 29 congenital Chagas cases, where 11 had hydrops (original article only available in Portuguese)
Congenital Trypanosoma cruzi Transmission in Santa Cruz, Bolivia, Bern, C., et al. (2009). Clinical Infectious Diseases, 49(11), 1667–1674.
Very complete discussion including good clinical, epidemiological and diagnostic information.
Hydrops fetalis in a congenital Chagas case in a non-endemic area, Teresa Gastañaga Holguera, Journal of Obstetrics and Gynaecology, 2016; Early Online: 1–2 [ISSN: 0144-3615 (Print) 1364-6893 (Online) Journal homepage: http://www.tandfonline.com/loi/ijog20]
Case report of a Bolivian woman living in Spain, hydrops fetalis was marked by subcutaneous and calvaria edema, ascites, hydrothorax and hydrocele. Peritoneal drainage performed, yielding ~500 ml/day. Serology and PCR positive for T cruzi. Benznidazole treatment given. In her discussion, author states that 60-90% of congenital infections are asymptomatic at diagnosis. From the author’s discussion section: “The most frequent clinical manifestations (of congenital T cruzi infection) are prematurity, low birth weight, jaundice, fever, oedema, anasarca, petechiae and myocarditis (Torrico et al. 2004). Anaemia, thrombocytopenia, leucocytosis or leucopenia, cholestasis, hypoalbuminaemia, hypertransaminasaemia and even coagulopathy can be observed.”
Congenital Chagas Disease: Recommendations for Diagnosis, Treatment and Control of Newborns, Siblings and Pregnant Women Yves Carlier, PLoS Negl Trop Dis. 2011 Oct; 5(10): e1250. open access
Not much clinical information. States that most cases of congenital Chagas are asymptomatic.
Toward Improving Early Diagnosis of Congenital Chagas Disease in an Endemic Setting, Louisa A. Messenger, Clinical Infectious Diseases, 2017;65(2):268–75
Fatal congenital Chagas’ disease in a non-endemic area: a case report, María Flores-Chávez, Cases Journal 2008, 1:302 doi:10.1186/1757-1626-1-302
Congenital Transmission of Chagas Disease — Virginia, 2010, Raul A. Lazarte, MD, et al, MMWR, July 6, 2012 / 61(26);477-479 open access.
Very good reference list.
Azogue, E.et al Congenital Chagas’ disease in Bolivia: epidemiological aspects and pathological findings. Trans. R. Soc. Trop. Med. Hyg.1985. 79, 176–180.
Not much clinical information regarding the affected infants. Describes striking ‘placental edema’…329 infants studied of whom 25 were positive for T cruzi (7.6%), maternal serological positivity was 51%, T. cruzi detected in 6% of placentas.
Maternal trypanosoma cruzi infection, pregnancy outcome, morbidity, and mortality of congenitally infected and non-infected newborns in bolivia, faustino torrico, Am. J. Trop. Med. Hyg., 70(2), 2004, pp. 201–209
In this population study, 3,800 mothers between 1999-2001 were studied and only 3 cases of fetal hydrops were detected. From the conclusion:”In conclusion, our study suggests that a decrease of poverty may reduce the morbidity and mortality, but not the transmission rate, of congenital T. cruzi infection. The latter remains an important risk for the babies of chronically infected mothers and a serious public health problem in Bolivia. Congenital T. cruzi infection is frequently associated with severe alterations in growth and maturity and neonatal death.”
Congenital transmission of Trypanosoma cruzi in central Brazil. A study of 1,211 individuals born to infected mothers, Alejandro O Luquetti, Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 110(3): 369-376, May 2015
This is a seroprevalance study with no clinical descriptions of the infected neonates. Congenital Chagas transmission rates average in the 5% range in South America. The authors note that very few cases of congenital transmission have been described in North America, despite the large number of potentially chronically infected mothers. (See above MMRW 2012 ref. that describes the first US congenital Chagas case.
A HYDROPIC CURIOSITY
from The New England Quarterly Journal of Medicine And Surgery. Boston, 1843. Dropsy of the Foetus, W. Channing. (Case V, page 328)
open access at: https://babel.hathitrust.org/cgi/pt?id=mdp.39015059415961;view=1up;seq=336
A case of a still-born dropsical foetus with a slightly oblique reference to the ‘theory of maternal impressions’ as an explanation of fetal pathology / teratology (Channing however, remains neutral in his clinical descriptions.) Recall the story of ‘the elephant man’, where John Merrick’s mother allegedly was frightened by a rearing elephant on a London street.
Dropsy of the Foetus, W. Channing,1843:
Case V. I know nothing of the labor in this case. During a winter term, a preparation was sent to me from the country, and described to be of very curious interest, as a monstrosity which had been produced by the imagination of the mother. The story was this. A sea-captain had returned from the West Indies, and brought with him a very large turtle. He had the animal carried to the house, and deposited him in the yard for safe keeping. His wife being then in the middle period of pregnancy, had not heard of the arrival of this guest, and encountered him as he was lying and sunning himself on the door-step. As she approached, he projected his limbs from the shell and moved off. She was exceedingly frightened. She went, however, her full time, and was delivered of a most misshapen mass, which was believed by every body to be the turtle. The weather was very cold, and for safe keeping, the monster being dead, was put into a room where it was soon frozen solid, and in this way preserved its shape perfectly. In this state it was sent to me. It soon began to thaw, and after remaining one night in my warm room in the college, I found in the morning that it was a dropsical foetus, entirely put out of shape, and I may add out of countenance, by the water which filled every part of it. The skin soon gave way, the water escaped entirely, and nothing remained but a loose bag of bones. I have stated this case as showing how extensively dropsy may exist in the foetus. Hydrocephalus, existing alone, has been referred to —then as complicated with ascytes—while this last case shows the universal occupation of the foetal body by water.
Christopher writes:
Good morning Professors,
It is a wet and cold 43 F here in Stonybrook long island. for this weeks case I would guess that the woman that Daniel saw was infected with Chagas disease. the parasite is commonly found in Bolivia, can go for long periods of time without detection and can cause pericardial diffusion according to this paper I found. https://www.ncbi.nlm.nih.gov/pubmed/17339570 so I think guess makes sense.
If the poetry book is still on the table I would like to be put into the running for it , if not well I guess Ill have to go buy it.
Lastly, I took some pictures of parasites from a 3 spined stickleback this week and because I know all of you enjoy pictures (especially Dickson) I thought I would share them with you. I’m not super talented with a camera so I apologize for the amateur quality, regardless I still think the pictures look cool!
Hoping to meet you all at the conference on DEC 3rd
Best,
Chris
Parasite pics in order

 pics 1 and 2 -> these pictures are of a Acanthocephalan parasite, a Male Neoechinorhynchus sp. found in the intestine approximately ~1mm in length
pics 1 and 2 -> these pictures are of a Acanthocephalan parasite, a Male Neoechinorhynchus sp. found in the intestine approximately ~1mm in length
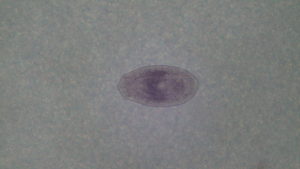 pic 3->tapeworm parasite specifically a Proteocephalus sp. also found in the intestine this one is about 3mm about I have found these to be anywhere from .5mm-20mm depending on development when caught. I normally find anywhere from 1 to 40 proteocephalus in each fish.
pic 3->tapeworm parasite specifically a Proteocephalus sp. also found in the intestine this one is about 3mm about I have found these to be anywhere from .5mm-20mm depending on development when caught. I normally find anywhere from 1 to 40 proteocephalus in each fish.
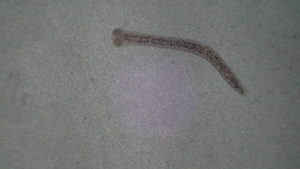 pic4-> this is a Diplostomum pseudospathaceum which is a trematode parasite. these are located in the eye of the fish and are about .5mm long. i normally find 1-12 of these in the eyes of the fish.
pic4-> this is a Diplostomum pseudospathaceum which is a trematode parasite. these are located in the eye of the fish and are about .5mm long. i normally find 1-12 of these in the eyes of the fish.
Hope you enjoy the pictures, sorry for not going to species level for all the parasites, we are still working on the stains for some of these to ID them further.
Chris writes:
Greetings TWIPsters,
It’s a typically wet and chilly start to the winter season in north Georgia, and the only pleasant place to be is indoors.
I’m most tempted to say the parasite affecting the child in this case was congenitally transmitted, because of the travel during pregnancy. And among likely infections, Toxoplasma seems the likeliest. If the mother has never been exposed to the parasite before, she will be unprotected and the tachyzoite stage can migrate to her fetus. The cardiac symptoms and ascites are consistent with Toxo; infections early in pregnancy produce more severe symptoms or even death.
The mother may have contracted this infection by accidentally consuming sporulated oocysts in the environment, or tissue cysts in under-cooked meat. Diagnosis can be made by serological testing, and the parasite can be treated in the child, but not completely cleared. For newborns, treatment with pyrimethamine, sulfadiazine, and leucovorin for a year is recommended.
All the best,
Chris
Athens, GA
Bening writes:
Greetings Tricksters,
I mean TWiPsters. It is sunny and 66F in Atlanta today. This episode’s case presentation of the newborn with cardiac issues and abdominal fluid threw me for a loop. I have never researched neonatal parasites outside of toxoplasmosis, so my first thought was: it’s toxoplasmosis! Right? Wrong…I think. Most congenital parasitic infections are asymptomatic and are difficult to diagnose, but after consulting PD6 pdf for congenital parasitic infections I was tempted to look further into congenital Trypanosomiasis cruzi. This was a strange choice seeing as it was the diagnosis of the last patient, but I could not shake the thought and ended up finding a case—which I will link below—that showed similarities in symptoms of ascites and pericardial effusion. Even more, the mother spent several months in endemic Bolivia prior to giving birth, as did our TWiP patient. While toxomplasmosis is a possibility, I will put my money on Trypanosomiasis. Diagnosis would best be achieved by microscopic examination of umbilical cord blood and testing the mother. She may have become infected while travelling to Bolivia or suffering from unknown chronic Chagas disease. Either case could lead to transmission to the baby. Benznidazole or nifurtimox have been shown to be effective treatments in congenital Chagas cases and would help prevent lifelong chronic infection. While sad, it is perhaps for the best that the child was symptomatic and treatment is an immediate option. The patient should be monitored post treatment to ensure the acute infection was treated completely and if not too advanced herself, the mother should seek to start treatment to prevent worsening chronic infection. I enjoyed this challenge and I am interested to hear the resolution of this case!
Sincerely,
Bening
Link to similar case:
https://academic.oup.com/jpids/article/5/4/e28/2631350
p.s. There does not seem to be a whole lot of information on congenital parasitic diseases and I had to dig around for quite a while to find even this much. Perhaps we could spend some more time in the future on these types of cases?
Lisset writes:
Dear TWiP professors,
Hello from Ann Arbor, Michigan currently at 36F/2C and snowing. I am an undergraduate student, taking Eukaryotic Microbiology for the first time who fell in love with parasitism since lecture one on Giardia. Our professor – shout out to him for his great work – recommended us to listen to your podcasts and now here I am challenging myself to email you guys. Anyway, here is my diagnosis for Case Study TWiP 160:
I think this patients is suffering from Chronic Chagas Cardiomyopathy. Chagas Disease (CD) results from infection with the hemoflagellate Trypanosoma cruzi. This cardiomyopathy is one of the late manifestations of heart involvement in CD. I found online that a left ventricular apical aneurysm is the most common echocardiographic abnormality seen in these cases. Typical findings include various heart blocks like right bundle branch block (like in our case), fascicular block, and complete heart block. To complete our diagnosis, we would need serological evidence of infection or tissue isolation of T. cruzi. I am thrown off by the fact that he already had an implantable defibrillator and was treated by a cardiologist BUT because the physician lived in a non-endemic areas this may have caused a misdiagnosis.
Please see attached the research paper that I used for all the fancy medical terminology and to find out the prevalence of T. cruzi in Brazil.
P.S: If I am wrong I am going to be devastated because I really wanted to get the book! By the way, I am with my classmate, who’s also a Microbiology major, and he thinks I am huge nerd for doing this.
Best,
Lisset
University of Michigan, 2019
Major in Microbiology | Minor in Spanish Lit. and Cult.
Barrett writes:
Drs. Racaniello, Despommier, and Griffin,
First let me thank you for such an engaging and informative podcast! I work in animal husbandry but have always had an intense interest in parasitology, especially fish parasitology, which has served me well working in the applied science of animal care in public aquaria and zoos.
Regarding the message on your last show regarding the paucity of female parasitologists as role models, I wanted to bring to your attention the contributions of Dr. Ida Mellen who was an ichthyologist and biologist at the New York Aquarium and later at Woods Hole in the early 20th century.
Dr. Mellen worked for many years at the New York Aquarium where her work laid the foundation for modern veterinary medicine of aquatic animals. She resigned without explanation in 1929, while we may never know the cause of her departure, reading the annals of the institution where the research exploits of her male counterparts were described in great detail, referring to the recent publication of “Dr. so-and-so” …we can only imagine her displeasure at having her own accomplishments minimized and attributed to “Miss Ida Mellen”. We can only speculate at the male-dominated culture she must have had to overcome as one of the nation’s first female marine science PhDs, but her accomplishments are still known in the fields of fish parasitology and veterinary medicine.
Arguably the most insidious metazoan parasite of marine fishes bears her name, Neobenedenia melleni, (Monogenea: Capsilidae) which is still problematic in public aquariums and aquaculture nearly a century after she discovered it on the eyes of aquarium fishes!
https://siarchives.si.edu/collections/siris_arc_306346
https://www.bklynlibrary.org/blog/2010/06/08/little-known-brooklyn
p.s.- an email on your last show mentioned a common saying in human medicine is also used in veterinary medicine, but you may find it interesting that “when you hear hoofbeats, think horses….” is also commonly used by taxonomists, I first encountered it in an ichthyology course in reference to identifying problematic specimens which are much more likely a common species than a rarity.
Barrett L. Christie
Director of Animal Husbandry
The Maritime Aquarium at Norwalk
Steve writes:
Hi Vincent et al.
How about branching out into plant and insect parasitism to broaden the podcast’s horizons?
This is the first I’ve heard of a plant seeking out insect structures to parasitise. Particularly interesting that its a parasite of a parasite too.
All the best,
Steve
Luton
England
Where the weather has dropped out of blazing summer just in time to give the standard cold and wet August Bank Holiday weekend!
https://www.livescience.com/63419-love-vine-drains-wasps.html
Anthony writes:
Dickson nailed it:
Dermatitis caused by Ctenocephalides felis (cat flea) in human
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4247491/
Once a day, I walked past some feeding stations setup for outdoor cats. I did not notice what was happening. Rather recently, a volunteer feeding the cats told me that “I think that my leg is covered with fleas.” I noticed that the distribution of the fleas matched the area of irritation on my leg. (I don’t know where cat fleas lurk, but it’s interesting that they just make there way to an area that’s feline height.) I checked my leg the next time I was outside and of course there were the little monsters. I never had seen any hint of fleas when I removed my socks in the evening. Presumably, the fleas hop off after eating — to annoy later the indoor cats and then die from Frontline.
We managed to get Frontline on a couple of the more approachable outdoor cats. I now spray with Pyrethrin as soon I get inside after walking by the cat feeding stations. The situation is coming under control.
